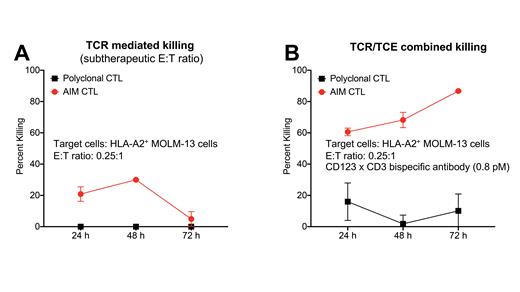
Multiple myeloma (MM) and acute myeloid leukemia (AML) are blood cancers that remain difficult to treat because a large proportion of patients have disease that does not respond to currently available therapies. NexImmune's Artificial Immune Modulation (AIM) platform mimics natural dendritic cell function by using nanoparticles engineered with MHC molecules loaded with tumor specific peptides (signal 1) and anti-CD28 (signal 2) for activation and expansion of HLA A*02:01 restricted tumor associated antigen (TAA)-specific cytotoxic CD8 T lymphocytes (CTL). Additionally, T cell engager (TCE) therapy allows for TAA-specific, but T cell receptor (TCR) independent T cell cytotoxicity mediated by immunological synapse formation between targeted cancer cells and T cells. Given that T cell function in MM and AML patients is often perturbed and the response to TCE therapy is determined by pre-existing endogenous T cells of the patient, we hypothesized adoptively transferred multi-antigen specific T cells with a rejuvenated phenotype in combination with TCE therapy might produce optimal efficacy. We compared polyclonal CTL with AIM CTL (CTL that were AIM primed and expanded for optimal antigen experience and specificity). We evaluated the preclinical efficacy of B cell maturation antigen (BCMA)xCD3 TCE+MM-specific AIM CTL combination therapy and CD123xCD3 TCE+AML-specific AIM CTL combination therapy both in vitro and in vivo. In co-culture assays with polyclonal CTL and AIM CTL, we investigated the effect of prior antigen exposure on the efficacy of the TCE against tumor cell lines by measuring the responses to T cells alone and in the presence of TCE treatment. We quantified tumor cell survival and thus determined cytotoxicity of the various culture conditions by luciferase assay. Our analysis showed that, as expected, CD4 T cells had little potency. Non-HLA matched control CTL (bulk or enriched for naïve cells) without TCE showed higher cytotoxicity (data not shown), but these cells were much less potent than AIM CTL (data not shown and Fig. 1A). Combined TCR and TCE-mediated target cell killing was most efficient when using AIM CTL as effector cells (Fig. 1B). Correcting the data for TCR mediated killing revealed that antigen-experienced AIM CTL were indeed superior effectors of TCE potency than antigen inexperienced control CTL. This finding was reproducible regardless of the HLA type of the target cells, consistent with the TCR independent mechanism of action of TCE-mediated killing. Nuclear factor of activated T cells (NFAT) transcriptional reporter assays with Jurkat effector cells and AML target cells revealed that TCE treatment decreased NFAT transcriptional activity in TCE effector cells; moreover, TCE treatment in vivo (treating mice with AIM CTL +/- TCE) increased CD3 levels on AIM CTL. Both observations are consistent with reduced TCR mediated T cell activation in the context of TCE therapy. Furthermore, BCMAxCD3 TCE treatment of mice in a MM xenograft model resulted in decreased PD-1 expression on AIM CTL and enhanced the in vivo efficacy of AIM CTL.
Our studies show that AIM CTL display strong anti-tumor activity, and even subtherapeutic AIM CTL cell doses can be highly efficacious when armed with a TCE. Importantly, our data indicate that AIM CTL cells are optimal TCE effectors, superior to less experienced, polyclonal CTL (such as the patient's endogenous T cells). Combining AIM CTL with TCE therapy has the additional benefits of complementary targets and mechanisms of action, reducing the risk of immune escape. Moreover, our preclinical studies suggest that TCE treatment reduces NFAT transcriptional activity and PD-1 induction associated with physiological TCR mediated T cell activation. This suggests that TCE mediated T cell activation can partially suppress TCR mediated T cell activation in AIM CTL, resulting in excellent tumor-specific cytolytic activity while inducing a transcriptional signature that preserves T cell fitness. Following withdrawal of TCE therapy, less exhausted AIM CTL will be present, facilitating the persistence of long-lasting anti-tumor activity and immunosurveillance. Combination of AIM CTL (autologous or HLA matched allogeneic) with TCE therapy is therefore a highly promising and innovative strategy for the induction of durable antitumor activity, and this approach has potential for clinical translation as a novel combinatorial immunotherapy.
Disclosures
Oelke:NexImmune Inc: Current Employment. Kim:NexImmune Inc: Current Employment. Wang:NexImmune Inc: Current Employment. Ragheb:NexImmune Inc: Current Employment. Zakrzewski:NexImmune Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal